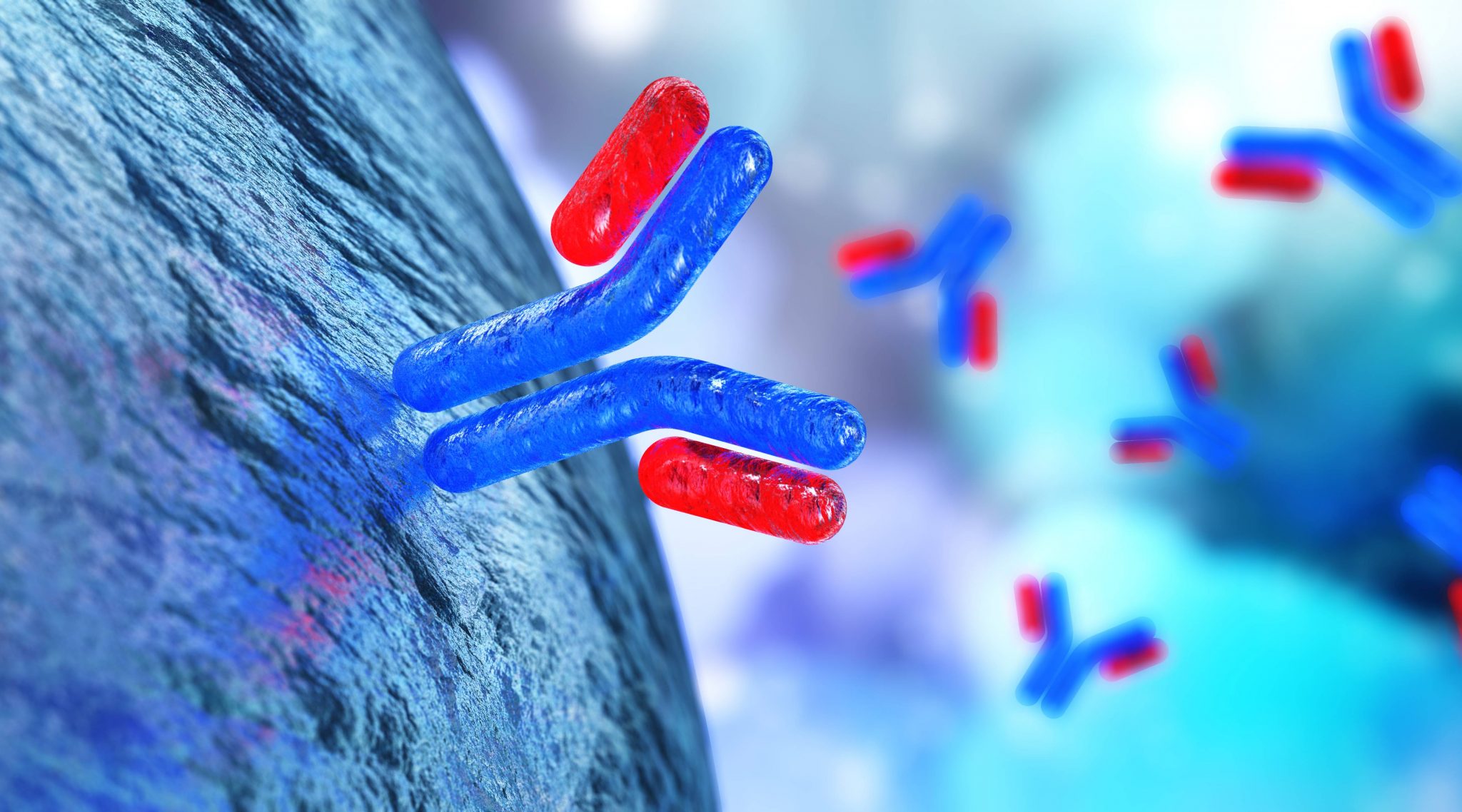
The fascinating field of antibody research was all but unknown just a decade ago. Today, drugs based on human and biosimilar antibodies constitute the quickest growing class of therapeutic agents. Read on to find out about key trends in antibody research to watch in 2019 and beyond.
Targeted Immunotherapy
Pharmaceutical engineering and biotechnology companies have been researching the use of antibodies for living organisms as a means of providing targeted immunotherapy. This approach has the lofty goal of using natural substances found in the human body or biosimilar substances engineered in labs to improve immune system function.
There are a few types of antibody-based immunotherapy currently in practice or undergoing research. They include monoclonal antibody therapy, non-specific immunotherapy, T-cell therapy, and others. Today’s antibody research focuses on different chronic diseases, including rheumatoid arthritis, but the most promising research pertains to finding new cancer treatments.
Antibodies and Cancer Treatment
Pharmaceutical companies have been manufacturing targeted antibody therapies for cancer patients for several years. Monoclonal antibodies work by blocking abnormal protein formation in cancer cells without affecting healthy cells. They also flag the cells so that patients’ immune systems can find and destroy them more easily.
Immune checkpoint inhibitors are also gaining popularity as effective treatments for cancer. They work by blocking patients’ PD-1/PD-L1 and CTLA-4 pathways, which cancer cells can use to escape detection by the patients’ immune systems. Blocking these pathways allows patients’ immune systems to find and respond to growing cancer cells.
Current antibody research focuses on tumor-agnostic treatments. Doctors can prescribe antibody therapies like pembrolizumab to treat metastatic tumors that are resistant to surgery. In order for antibody therapies like pembrolizumab and others to be effective, patients’ tumors must have specific genetic changes that cause them not to repair DNA damage well.
Increased FDA Approval of Biosimilar Products
The US Food and Drug Administration (FDA) started approving biosimilar products of antibody therapeutic drugs four years ago. In March 2019, the FDA approved 16 new biosimilar antibody therapies. They include biosimilars of five reference products: trastuzumab, adalimumab, infliximab, bevacizumab, and rituximab.
Current Studies
Sandoz is currently running a Phase 1/3 clinical study to compare the pharmacodynamics, pharmacokinetics, immunogenicity, safety, and effectiveness of a proposed biosimilar of denosumab called GP2411 that could be used in the future to treat osteoporosis in postmenopausal women. Samsung Bioepis Co., Ltd., is currently performing a Phase 3 evaluation of a proposed ranibizumab biosimilar to Lucentis, referred to as SB11, for use in treating neovascular age-related macular degeneration that will be completed in November. Formycon and Bioeq IP AG submitted an application to the FDA for another biosimilar of Lucentis FYB201, with the intention of beginning to market it in the US in 2021.
The Bottom Line
Antibody therapies provide safe and effective solutions for patients with chronic illnesses ranging from rheumatoid arthritis to macular degeneration and even cancer. The field of antibody research is still in its infancy, though. It’s worth paying attention to what’s happening now and what experts in the field expect to happen next.
Doctors, pharmacists, and scientists can expect to see many advances in this revolutionary field in the coming years as the FDA continues to approve new therapeutic drugs. Patients can expect these drugs to become more accessible as additional biosimilars gain FDA approval and researchers discover new ways to take advantage of antibody therapies to create new cures.